Pharmacovigilance -- "Living Reviews" Applied
As Dr. Jon Brock discussed last month in Nature, there is a fundamental obstacle when conducting any document review, especially systematic reviews: new information.
The purpose of every review, regardless of its protocols, is to identify relevant information, organize and/or extract the data, and (hopefully) find a meaningful conclusion. As Dr. Brock describes, the issue is that a constant production of new information means that by the time any conclusion is drawn, the study is outdated. Researchers must be able to constantly digest new information into ever-adjusting conclusions.
"Living reviews" are an adaptation of classical review-based research that attempt to facilitate just that. The idea is that as new information is produced, it is immediately funneled into the review pipeline and evaluated by the exact same protocols as the previously existing data. The new (validated) data is incorporated and conclusions adjusted as necessary. Sounds simple - but not quite so in practice.
What happens if new information directly repudiates existing data? What happens when new studies suggest that the evaluation criteria and protocols that the review has been using are flawed? What happens when the researchers realize there is a critical piece of information within the studies that has been ignored?
Not only must a living review consider new information, it must consider its own assumptions and foundations. Moreover, it needs to do so in an intelligent, and transparent, fashion. Nowhere is this more true than in pharmacovigilance.
According to the World Trade Organization, pharmacovigilance is the science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other possible drug-related problems.
What that means in practice, is that organizations conducting pharmacovigilance must constantly consider all sorts of new types of information: toxicological studies, clinical trials, adverse outcome reports, and even market information. For each type of information, there are likely many sources – each with their own reporting style – all of which must be considered in a standardized way.
The solution, then, is not simply a living review, but a growing repository of information - fed by an ever increasing number of data streams - that informs any variety of processes and applications. This repository must also facilitate the review, and if necessary amendment, of prior work – all the while logging any and all changes.
The Sysrev platform is such a solution. The most important aspect of pharmacovigilance is considering all the relevant information in one's conclusions. Users of Sysrev are able to create as many projects as they desire which provides upmost flexibility in organizing different data streams.
Within a single project, users can upload as many batches of documents as they need – tracked of course by Sysrev. For on-going, consistent reviews, Sysrev can even incorporate new data streams directly into the platform. Talk to us about our enterprise solutions to learn more.
Sysrev not only has the bandwidth to organize pharmacovigilance documents but the technical features to analyze them in an efficient manner.
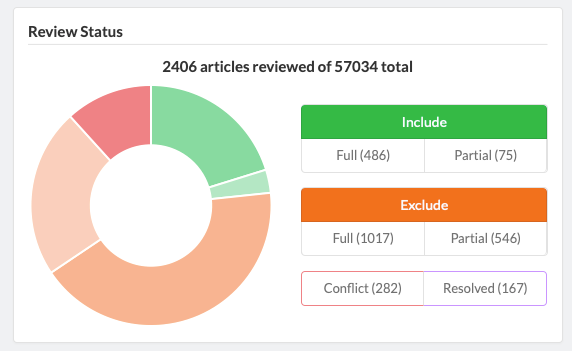
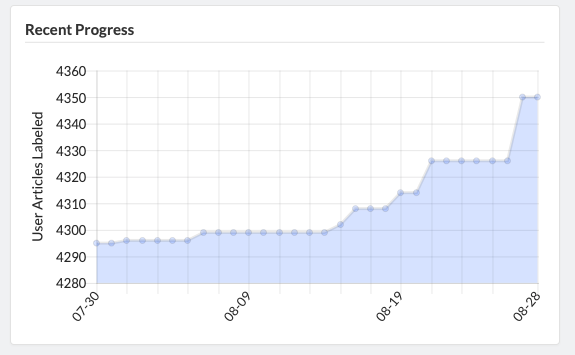
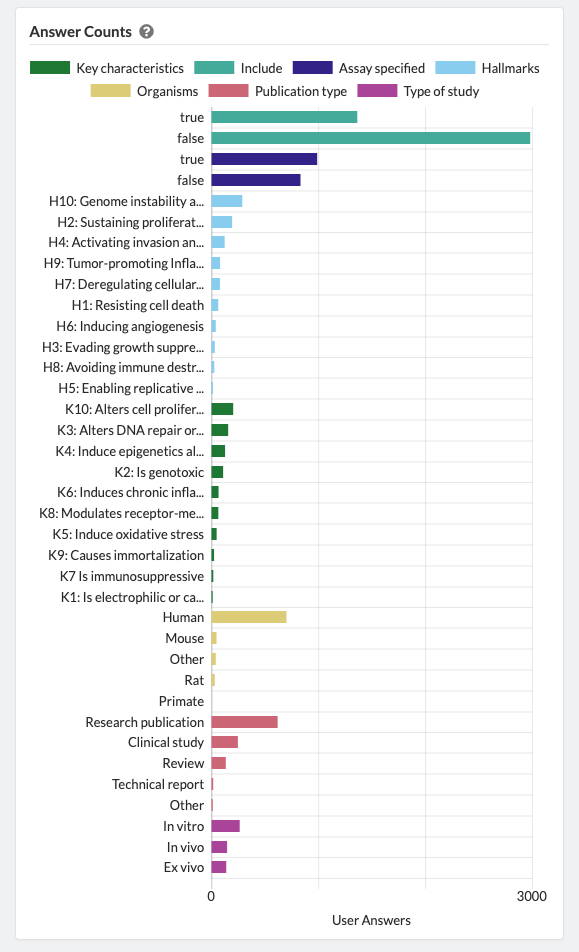
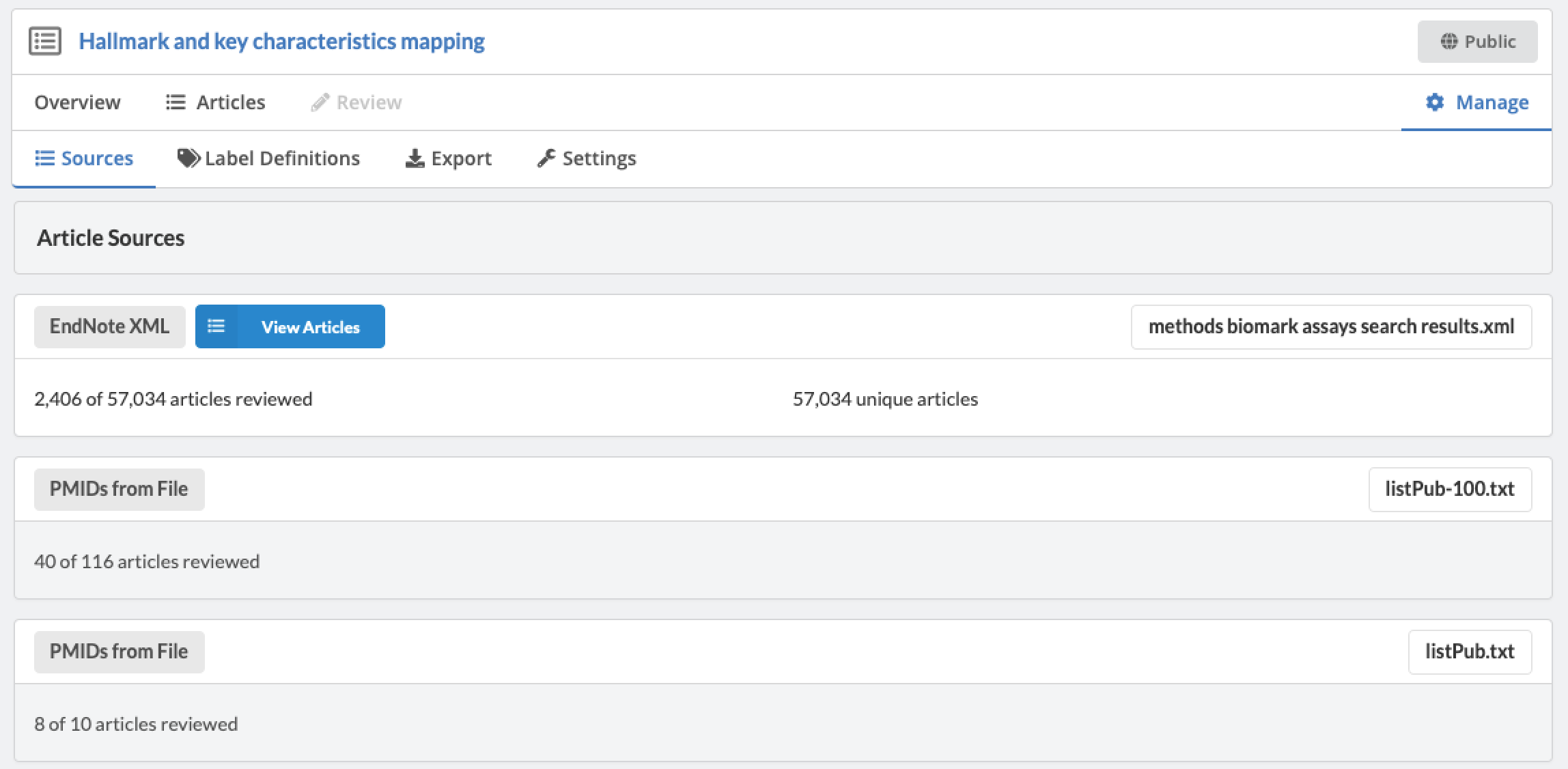
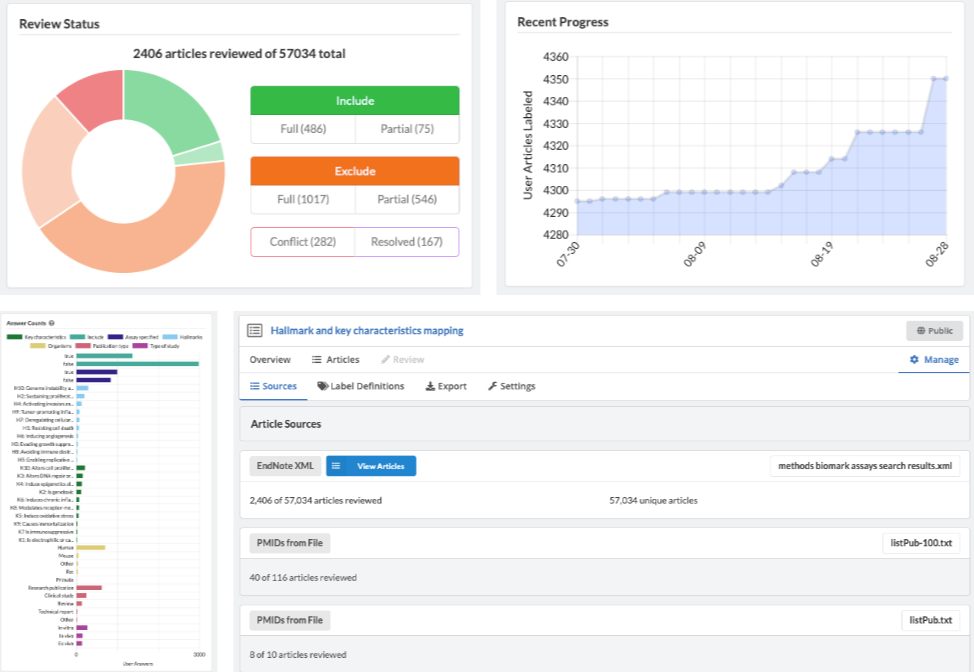
Admins are able to define inclusion/exclusion criteria, as well as manage which data is being extracted. The Admin Dashboard gives the project manager the information they need to evaluate the progress of the review, including user and label concordance.
Projects can easily be cloned for a variety of reasons: record keeping, amending past work, extracting new data from existing sources, or adding new sources of information to be considered. Sysrev tracks the flow of sources and data through the entire review pipeline. As shown below for the "Hallmarks and Key Characteristics Mapping" project, Sysrev even learns to predict which documents should be included or excluded. With sufficient training, Sysrev can vastly reduce the time associated with reviews, by minimizing time spent on irrelevant or non-qualifying information.
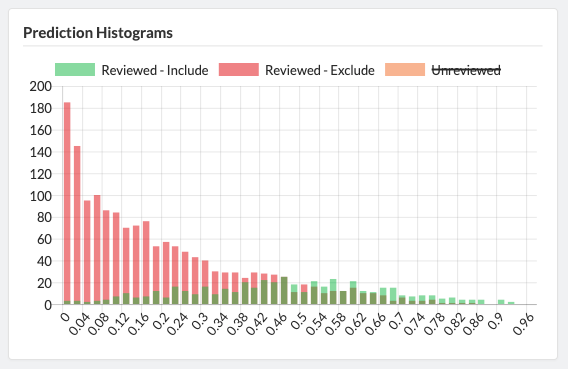
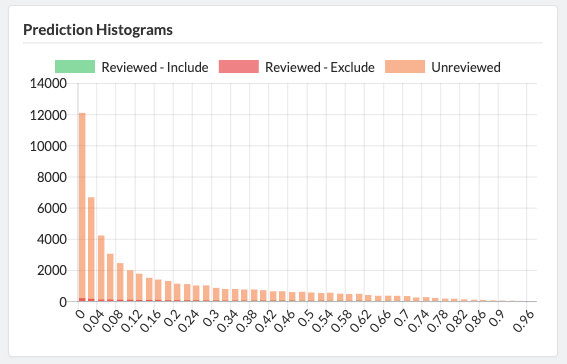
The final extension of the Sysrev platform is automation. When review tasks are sufficiently standardized, such as extracting information from an MSDS... or when the extracted data is used in sufficiently standardized way, such as filling out a ICSR E2B(R3)... there is an opportunity to utilize artificial intelligence to automate the task. Contact us to learn more about developing custom artificial intelligence applications for your enterprise.
